Flotillin-1 palmitoylation is essential for its stability and tumor-promoting functions in triple-negative breast cancer (TNBC). Disrupting this process, either genetically or with targeted inhibitors, reduces tumor growth and metastasis, making it a potential therapeutic target.
Background
Triple negative breast cancer (TNBC) is a particularly aggressive form of breast cancer that lacks targeted therapies, leaving patients primarily reliant on highly toxic chemotherapeutic treatments. The high rate of metastasis associated with TNBC significantly contributes to its mortality rate, necessitating the identification of novel therapeutic targets.
Flotillins, particularly flotillin-1, are proteins enriched in lipid rafts within plasma membranes and are implicated in stable signaling platforms for receptors, promoting intracellular trafficking, stability, and signaling. Their expression is upregulated in many solid tumors, correlating with increased invasiveness and metastasis. Flotillin-1 undergoes several post-translational modifications, including palmitoylation, which is crucial for its stability and function.
However, the exact mechanisms by which flotillin-1 palmitoylation influences its role in cancer progression remain poorly understood. Current approaches to targeting TNBC are inadequate, as they do not specifically address the molecular pathways involving flotillin-1, highlighting the need for strategies to disrupt its tumor-promoting activities.
Technology description
Flotillin-1 palmitoylation is crucial for its stability and tumor-promoting functions in TNBC (see Figure 1). By creating TNBC cell lines expressing a palmitoylation-defective form of flotillin-1, it was demonstrated that the absence of palmitoylation leads to proteasomal degradation of flotillin-1. The enzyme zDHHC5 was identified as the primary palmitoyl acyl transferase responsible for this modification.
In vivo experiments using mice showed that cells with palmitoylation-defective flotillin-1 exhibited reduced tumor growth and lung metastasis. Additionally, a competitive palmitoylation inhibiting peptide was designed and validated, showing potential as a therapeutic target by reducing flotillin-1 stability and subsequently decreasing its tumor-promoting activity. The study also found that flotillin-1 palmitoylation status affects flotillin-2 levels, another protein involved in tumor progression.
This technology is differentiated by its focus on the palmitoylation of flotillin-1, a modification essential for its stability and tumor-promoting capabilities. Unlike traditional therapies that target broader mechanisms, this approach specifically disrupts the palmitoylation process, leading to the proteasomal degradation of flotillin-1.
The identification of zDHHC5 as the main palmitoyl acyl transferase and the development of a competitive palmitoylation inhibiting peptide provide a targeted therapeutic strategy. This specificity not only reduces the stability of flotillin-1 but also impacts flotillin-2 levels, thereby offering a multi-faceted approach to inhibit tumor growth and metastasis in TNBC.
The in vivo validation of reduced tumor growth and lung metastasis further underscores the clinical potential of targeting flotillin-1 palmitoylation.
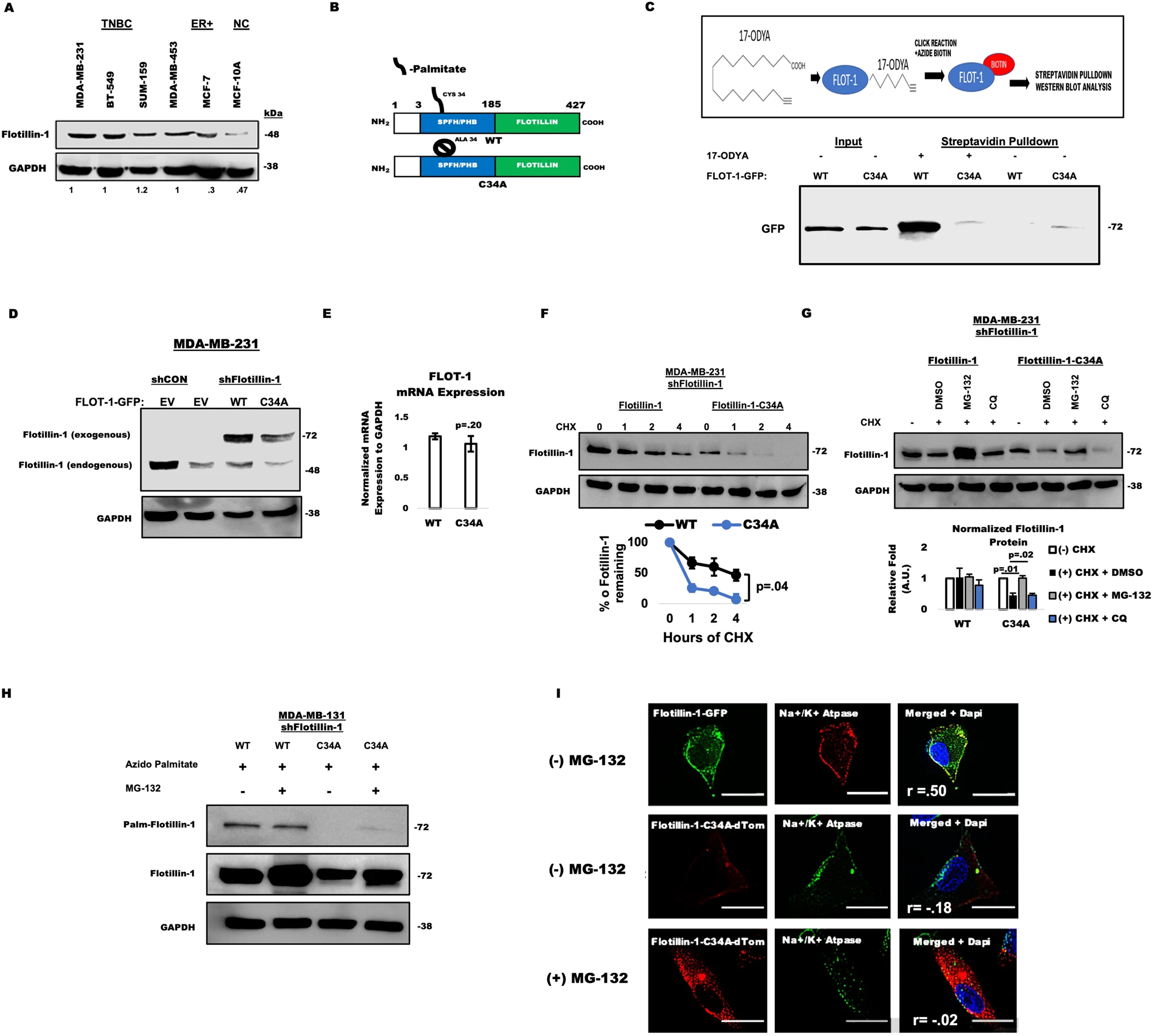
Figure 1.
A: Flotillin-1 expression in TNBC and control cell lines; densitometry results shown (n=1).
B: Schematic of WT and C34A flotillin-1 constructs.
C: Click chemistry detection of palmitoylated flotillin-1 in HEK293T cells; experiments repeated twice.
D: Flotillin-1 expression analysis in MDA-MB-231 cells with various constructs; conducted twice.
E: Flotillin-1 mRNA levels in WT vs. C34A MDA-MB-231 cells; n=3 biological replicates ±SD.
F: CHX chase assay for flotillin-1 stability in MDA-MB-231 cells; n=2 independent trials ±SD.
G: Western blot analysis of flotillin-1 after CHX treatment; mean ± SEM from two trials (n=2).
H: Detection of flotillin-1 palmitoylation in stably expressing cells with MG-132; repeated twice.
I: Immunofluorescence for flotillin-1 localization with Na+/K+ ATPase; co-localization quantified using Pearson correlation (3 independent experiments).
Benefits
- Identification of zDHHC5 as the primary palmitoyl acyl transferase for flotillin-1
- Better understanding of flotillin-1 and -2 interaction in tumor progression
- Development of TNBC cell lines expressing palmitoylation-defective flotillin-1
- Validation of flotillin-1 palmitoylation status affecting flotillin-2 levels
- Reduction in tumor growth and lung metastasis in TNBC
- Potential therapeutic target for TNBC through competitive palmitoylation inhibition
Commercial applications
- Cancer therapeutics
- Protein stability assays
- Drug development
- Biomarker identification
Patent link
WO2023250520A2

