Problem
In the pursuit of optimizing the delivery of brain-penetrating drugs, researchers have explored innovative technologies to enhance their accumulation within the central nervous system (CNS). Traditional strategies such as altering drug chemistry or formulation have shown limited success in extending patient survival, particularly in the context of glioblastoma (GBM) treatment with temozolomide (TMZ). Traditional therapies such as chemotherapy and radiation often fail to adequately target and eradicate GBM cells, leading to poor patient outcomes. Existing delivery systems for therapeutic agents, particularly virus-based approaches, face challenges such as limited payload capacity and safety concerns.
Target market
There are currently no effective treatments for glioblastoma—a diagnosis today is always fatal, generally within a year or two. Current efforts are focused on getting more drug into the brain, not on keeping it there longer. The global glioblastoma multiforme treatment market size was estimated at USD 2.46 billion in 2022 and reached USD 2.67 billion in 2023.
Solution
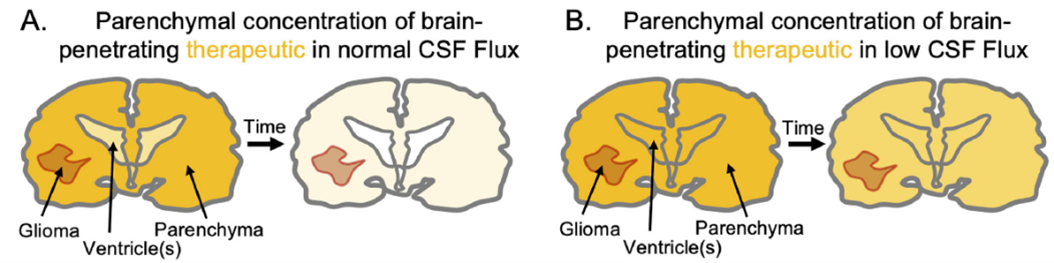
To overcome these challenges, researchers at The University of Texas at Austin discovered a novel approach, focusing on modulating cilia to alter fluid dynamics in the brain to enhance drug accumulation. Early tests in mice show significant promise. Normally, brain-penetrating drugs quickly clear from the CNS. Slowing CSF flux reduces drug clearance, increasing drug accumulation in the brain over time and enhancing its interaction with tumors (Figure 1B). This approach offers a revolutionary change in how we treat brain diseases like glioblastoma, offering hope for better outcomes and potentially opening doors to new treatments for various neurological conditions.
References
Umlauf BJ, Frampton G, Cooper A, Greene HF. A novel strategy to increase the therapeutic potency of GBM chemotherapy via altering parenchymal/cerebral spinal fluid clearance rate. J Control Release. 2023; 364:195-205.

