All-solid-state batteries are the next logical step to improve upon the Li+-ion battery for energy storage in portable electronic device. With the desire to advance the per charge driving range of electric vehicles, improvements to the volumetric and gravimetric energy density of the battery packs must be made. The most-direct method of improving the energy density over the current Li+-ion battery is to use a lithium metal anode in place of the usual graphite anode. However, lithium metal is unstable upon charge/discharge cycling in the typical commercial organic liquid electrolyte with a propensity to form lithium dendrites. These dendrites can grow across the battery and cause a short circuit that can catalyze thermal runaway, leading to catastrophic failure of the cell. A direct avenue towards enabling a lithium metal anode and improving the energy density of rechargeable lithium batteries by up to 10x is by replacing the current commercial organic liquid electrolyte with a solid electrolyte. To date, no solid electrolyte has demonstrated all the necessary requirements to serve as a drop-in replacement for current Li+-ion batteries, but solid polymer electrolytes and composite polymer electrolytes are two of the most promising options currently available.
Researchers at The University of Texas at Austin have developed a technology to improve solid polymer electrolytes and composite polymer electrolytes, enabling an all-solid-state battery with a commercially competitive cathode and a lithium metal anode. Figure 1 demonstrates the performance of full coin cell batteries with LiFePO4 and LiNi0.8Mn0.1Co0.1O2 (NMC-811) at 55°C. These all-solid-state batteries show excellent cycling stability and energy efficiency.
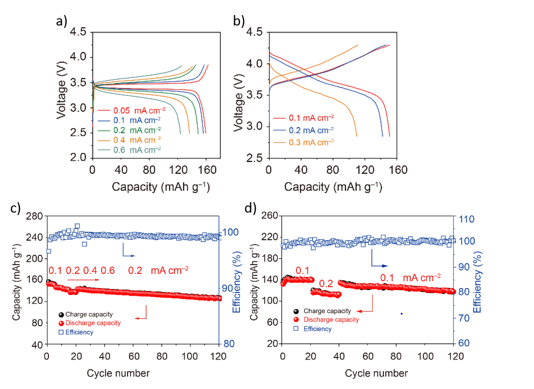
Figure 1. Full all-solid-state battery performance at 55˚C of batteries consisting of a) LiFePO4 and b) LiNi0.8 Mn0.1 Co0.1 O2 cathodes and a lithium metal anode with a composite polymer electrolyte utilizing the low-cost additive from this invention.

