Background
Light olefins are produced in greater quantity than any other organic chemical, with over 200 million metric tons of ethylene and propylene generated in 2016—roughly 30 kilograms for every person worldwide. Currently, olefin-paraffin separation, essential to the process, is performed by cryogenic distillation at elevated pressures. This separation is particularly difficult because the thermophysical properties of olefin-paraffin pairs are very similar, and no alternative technologies have been commercialized. Olefin-paraffin separation alone is estimated to account for 0.3% of total global energy use. Membrane processes are inherently energy-efficient because they do not require adding or removing thermal energy to induce a phase change. They are also low-footprint, modular, and uniquely suited for applications where large distillation columns are not feasible.
Technology description
Researchers at The University of Texas at Austin have developed a membrane which allows for olefin-paraffin separation while maintaining this selectivity even after hydrogen permeation. Silver ion facilitated transport membranes have the necessary high selectivity, but they rapidly lose selectivity upon exposure to reducing agents present in the industrial process, such as trace levels of hydrogen. Previous studies and technology showed a rapid decline in and near total loss of selectivity after one week of hydrogen permeation. Degradation of silver ion containing membranes by hydrogen is reported to be a key obstacle to the commercialization of this class of polymeric membranes for olefin-paraffin separation. The invention has demonstrated high pure gas ethylene-ethane selectivity even after repeated exposure to very high levels of hydrogen relative to those found in the targeted industrial application.
Results
The membrane developed is chemically stable to hydrogen, a reducing agent often present as a byproduct of industrial olefin production. As shown in Figure 1 below, the selectivity of olefin-paraffin remains constant, even in the presence of hydrogen. The pure-gas ethylene-ethane selectivity is much less sensitive to long-term hydrogen exposure than other such membranes reported in literature. Long-term hydrogen stability studies were conducted at two different pressures of hydrogen, with the longest experiment lasting over ten weeks without a substantial decrease in ethylene-ethane selectivity.
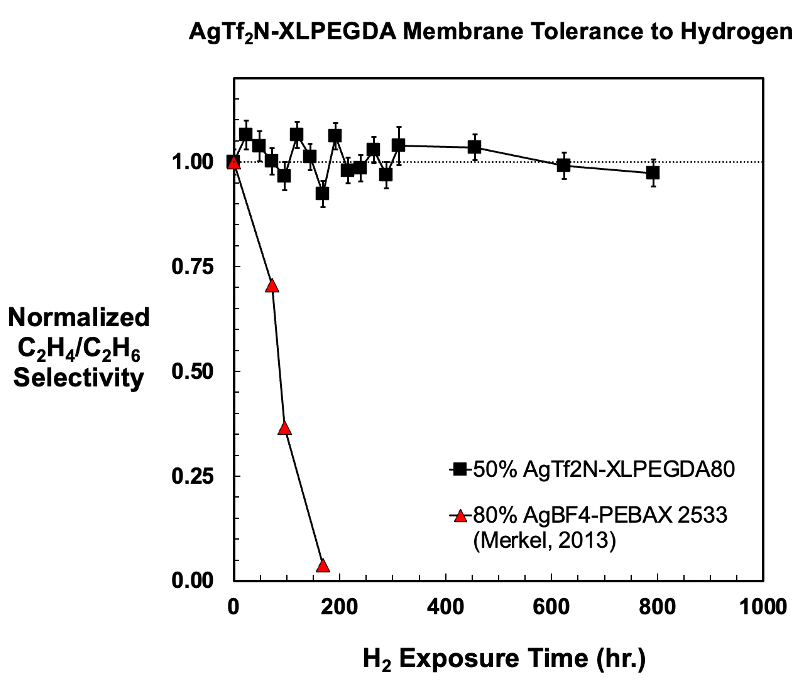
Figure 1. Olefin-paraffin selectivity versus hydrogen exposure time.

