Lithium chloride (LiCl) is a critical lithium containing material involved in producing battery-grade lithium hydroxide (LiOH). LiOH is required as a precursor material for many of the high-performance Li+-ion battery cathode chemistries in use today for portable electronic applications from laptops to electric vehicles. However, current battery production is already beginning to push the limit of readily available lithium reserves from traditional mining techniques. Some estimates predict readily available lithium resources will not be able to meet global demand as early as 2023. Although there is great effort being put towards developing lithium recycling technology, the current global rate of lithium recycling does not greatly aid the situation, as it is less than 1%.
Researchers at The University of Texas at Austin have developed a new material that can selectively remove LiCl from multi-salt systems via a solid-liquid or liquid-liquid extraction process. This material allows for LiCl to be harvested from non-traditional, more sustainable supply sources, such as brackish brines. A proof of the effectiveness of this technology is shown in Fig. 1, which shows spectroscopic results of this material after the extraction process from solid salt mixtures. These results illustrate the drastic selectivity of these materials in preferentially extracting lithium chloride over sodium or potassium chloride in a solid salt mixture.
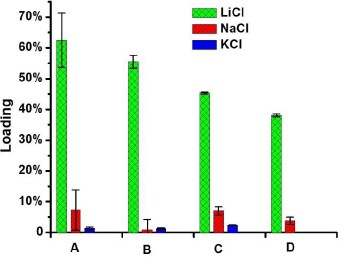
Fig. 1: Analyses of salt loading in novel lithium salt extraction materials, showing the percent loading of the indicated alkaline chloride salt after the extraction process from solid mixtures of (A) LiCl, NaCl and KCl (100:100:100, molar ratio), (B) NaCl and KCl containing 10% of LiCl, and (C) analogous experiments where the LiCl content was 1%, the concentration of extractant was 4 mM in nitrobenzene in all three studies. (D) Analogous studies involving a solid mixture of NaCl and KCl containing 200 ppm of LiCl 3 mM of extractant in chloroform. All percentages provided are by mass content unless otherwise noted.

